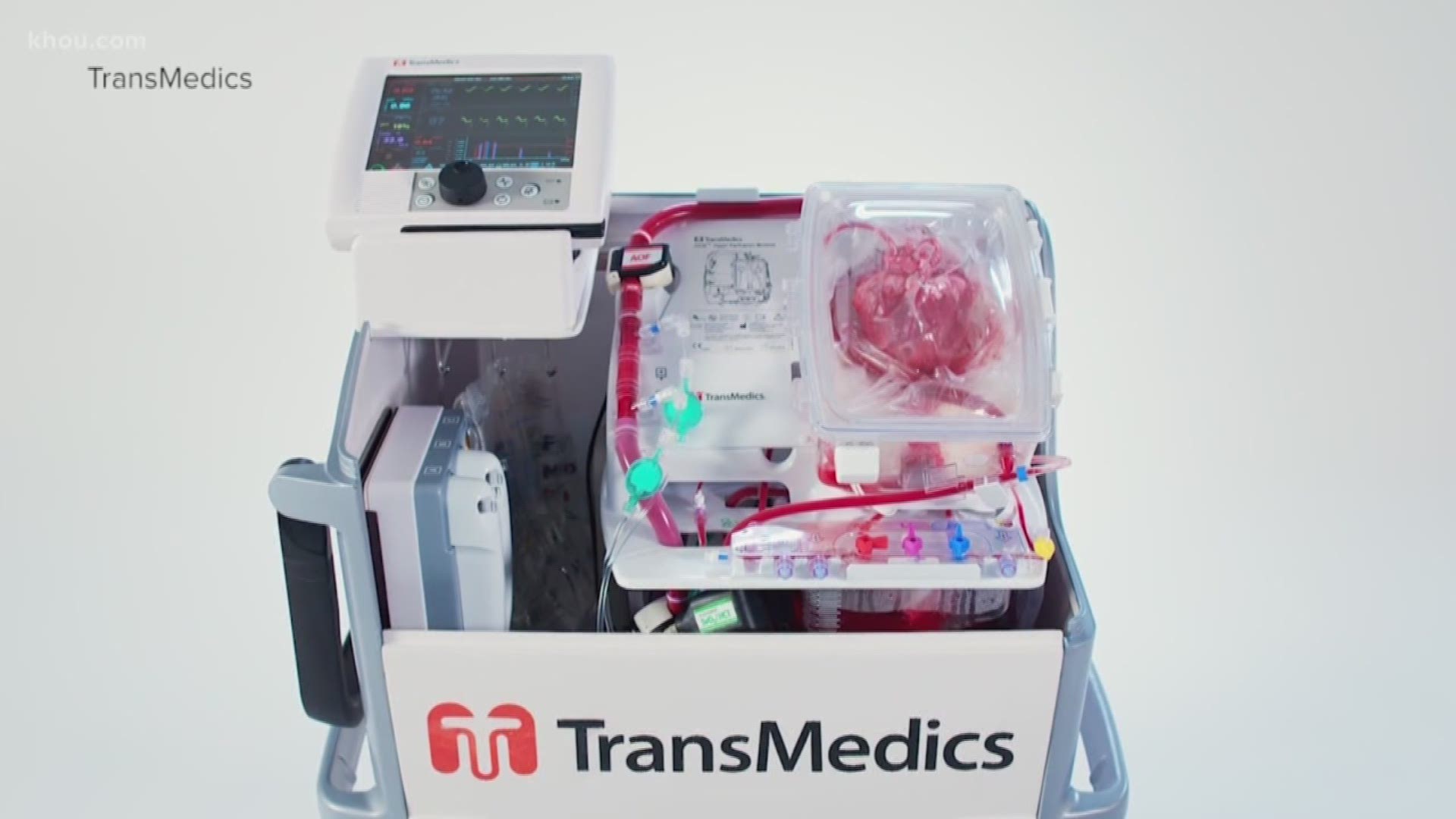
HOUSTON — A new device that was tested in Houston is revolutionizing lung transplantation.
The TransMedics Organ Care System, or breathing lung device, simulates human-like conditions for the donor lung while in transit.
Baylor St. Luke’s Medical Center was the first hospital in Texas -- and third in the country -- to successfully complete a breathing lung transplant.
The FDA approved the technology for standard use in June.
“Instead of being limited to three hour windows, for instance, you can go out as far as 12 hours, or even more,” said Dr. Gabriel Loor, director of lung transplantation at the Baylor College of Medicine.
Dr. Loor said they’ve done over a dozen breathing lung transplants.
The clinical trial showed lungs transported in the device woke up twice as fast after transplant and had excellent one-year survival rates.
Jackie Cundiff underwent a breathing lung transplant in the Spring. She was able to go home one week after surgery.
“If you’ve never struggled to breathe, you don’t realize how glorious it is to be able to breathe again,” Cundiff said. “I’ve done a lot more with the kids since transplant. I’m only nine weeks out, so we’re planning bigger.”
The people behind the Organ Care System are planning bigger, too.
Clinical trials are underway to use the device for liver transplants. Doctors are also studying whether it could work for heart transplants.
ALSO POPULAR ON KHOU.COM